This article was originally published by The Defender – Children’s Health Defense’s News & Views Website
Summary:
- The U.S. Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC) cooperated to issue Emergency Use Authorizations (EUA) and roll out new, bivalent Pfizer and Moderna COVID-19 vaccines this week, without any human trials, which is unprecedented.
- There is international coordination regarding bivalent boosters, and a major effort will be undertaken to get them into arms, despite historically low levels of severe COVID-19. Why?
- These vaccines continue to enjoy extraordinary protection from liability, while the recipient has no access to the legal system in the case of injury.
- There is no evidence the new vaccines are safe, while there is limited evidence that they may be more harmful than earlier COVID-19 vaccines. However, in the absence of human testing, there is no way to truly predict their safety. Safety data are being concealed by federal health agencies. Messaging by them is misleading.
- There is no evidence the new bivalent vaccines will be more effective than the older vaccines, and existing evidence suggests that any efficacy they provide will persist no longer than one to several months.
- COVID-19 vaccines appear to increase susceptibility to COVID-19 infections, on average starting six months after inoculation.
- Perpetual boosters briefly stave off the negative efficacy that develops a few months after a COVID-19 vaccination. This may be why frequent boosters are being pushed. But frequent boosters may also weaken overall immunity and may even contribute to rising mortality rates in the U.S. and U.K.
(Children’s Health Defense) – The FDA on Aug. 31 issued EUA for new Pfizer and Moderna mRNA booster vaccines for COVID-19.
One day later, the CDC and its director, Dr. Rochelle Walensky, approved the immediate rollout of the new vaccines, which will be administered in the U.S. beginning this week.
Surprisingly, more than a month before either agency had given its okay to the entirely new formulation, the federal government ordered 105 million doses from Pfizer and 66 million doses from Moderna.
The desired composition of the vaccine had only been formally determined by the FDA after its advisory committee met on June 24.
The vaccines contain a mix of the old, original Wuhan strain vaccine mRNA (now also referred to as the ancestral vaccine) and a new Omicron BA.4/5 mRNA coding for the Omicron spike protein.
The total amount of mRNA for the Pfizer and Moderna booster vaccines is the same as before: 30 mcg for Pfizer and 50 mcg for Moderna. Each is composed of 50% Omicron mRNA and 50% ancestral mRNA, and thus they are termed bivalent vaccines.
The new vials and their boxes do not list the dose, hinting that the decision regarding how much to use was made very recently. Even the members of the CDC’s advisory committee did not know the dosage of the new bivalent vaccines until their Sept. 1 meeting.
This is the fastest rollout of a new vaccine in world history. And instead of this being a tale of human grit and ingenuity, it is a tale of human weakness and recklessness.
How did such a rapid vaccine rollout occur?
It occurred the only way it could possibly occur: by bending the rules, creating a new regulatory playbook and failing to obtain any human data for the new vaccines.
The manufacturers did not have to go through months-long trials, and the FDA did not have to pore over any human trial data, because there weren’t any.
Let that sink in: The new BA.4/5 bivalent vaccines were tested only in mice, not humans.
Unexpected international coordination
Here is an amazing fact: On Sept. 1, the same day the CDC approved the vaccine program, health agencies in Canada, Switzerland and the European Medicines Association (the EU’s equivalent to the FDA) also rolled out new, bivalent booster shot programs.
Almost simultaneously, the U.K. authorized two different bivalent boosters, on Aug. 15 and Sept. 3.
The U.K. told people to expect the largest rollout in history for the new bivalent boosters — and it started the program by promising large bonuses to doctors if they manage to vaccinate every single resident of a nursing home by Oct. 23.
These other countries are using an earlier Omicron mRNA as the template for their Omicron-ancestral bivalent vaccines, while the U.S. is using the mRNA code for the later Omicron variant BA.4/5 spike.
Mainstream media skirts key questions
How are the mainstream media telling this story? With their usual spin — avoiding the sticky parts.
Instead of helping you understand what just happened, The New York Times asks, “When should you get yours?” Not should you get it, just when should you get it.
The Herald Tribune tells you why you should get it. STAT News says it answers your questions, but it never asks the relevant questions about why such a rapid, unprecedented rollout occurred — especially when we are at practically historic lows for deaths and ICU stays due to COVID-19.
The Associated Press did slightly better, at least posing the question of whether you should get a new booster — but then its answers don’t dig any deeper than its fellow media outlets.
It looks like you won’t be getting the information you need to understand the boosters and the process by which they were ushered in from the major media.
So The Defender reviewed FDA documents, attended the all-day CDC advisory committee meeting on Sept. 1, studied a review of the boosters published Aug. 31 in the New England Journal of Medicine (NEJM) and evaluated a study of Omicron boosters that were tested in non-human primates by Dr. Anthony Fauci’s Vaccine Research Center.
FDA spins ‘safe and effective’ narrative despite lack of supporting data
The FDA did not convene its advisory committee before issuing the authorizations — it’s not hard to guess why.
Last year, FDA advisors voted against authorizing the ancestral boosters because the data they were given indicated the old vaccines were working well.
Two top FDA officials who disagreed with the 2021 booster rollout resigned, hinting the decision to issue boosters had been imposed on the FDA.
This year, the FDA’s Vaccine and Related Biological Products Advisory Committee (VRBPAC) members have been complaining about being given less and less data as they are asked to sign off on vaccine programs for younger and younger ages.
VRBPAC member Dr. Paul Offit, a professor of pediatric infectious diseases at the University of Pennsylvania and coinventor of a rotavirus vaccine, last month said, “The fix was in,” implying the committee’s deliberations were a sham and noting the White House announced it was purchasing the vaccine right after the meeting ended.
Offit last week said the mouse data were not sufficient to roll out the new boosters. So the FDA chose not to give him and the other members a public venue where they would predictably complain about FDA’s laxity — what some might call recklessness, insubordination or even gross malfeasance.
After all, according to the FDA’s mission statement, FDA “is responsible for protecting the public health by ensuring the safety, efficacy, and security of human and veterinary drugs, biological products, and medical devices” — not rubberstamping untested vaccines.
The FDA justified its authorizations using language that was probably intended to confuse the public.
For example, everyone knows the term “safe and effective,” which is an official FDA stamp of approval for licensed drugs and vaccines.
However, by law the term cannot be used by the FDA to refer to unlicensed, experimental products, which is what all EUA drugs and vaccines are.
So in a press release on the new boosters, the FDA used almost, but not quite identical terminology, quoting Dr. Peter Marks, the director of the FDA’s vaccine center: “We have worked closely with the vaccine manufacturers to ensure the development of these updated boosters was done safely and efficiently.”
Marks also said, “The public can be assured that a great deal of care has been taken by the FDA to ensure that these bivalent COVID-19 vaccines meet our rigorous safety, effectiveness and manufacturing quality standards for emergency use authorization.”
Again, “safe and effective” is implied but not exactly stated.
What Marks expects the public to miss is the fact that there are no quality standards for EUAs. The statute authorizing EUAs simply requires that the known and expected benefits outweigh the known and expected risks of the product.
The FDA is not even required to inspect the factories where EUA products are manufactured, as it must do for licensed products. Nor is it required to inspect the final product.
Marks and the FDA know that all EUA products have been granted an extremely broad waiver of liability that covers Marks, the FDA, CDC, U.S. Department of Health and Human Services, the vaccine manufacturers and distributors, doctors, pharmacists and everyone involved in the vaccine program.
So they can tell us anything because the public has no recourse to the courts to bring suit when an EUA product is involved.
The FDA justified its assessment that the untested vaccines are safe using the following argument: “The safety data accrued with the bivalent vaccine (original and Omicron BA.1) and with the monovalent Moderna COVID-19 Vaccine are relevant to the Moderna COVID-19 Vaccine, Bivalent because these vaccines are manufactured using the same process.”
This is the same as claiming that almond butter is safe, so peanut butter is safe, too, because it is manufactured using the same process.
Is that really the best excuse for failing to perform the regulatory functions that the FDA can offer?
Sept. 1 ACIP meeting: boosters for any adult who wants one, and soon for kids
The CDC knew that it would have a hard time convincing the public to take these vaccines, as almost everyone has already had COVID-19, the earlier vaccine benefits were overpromised, the disease has become milder, the vaccines do not prevent infection or transmission and the fearfulness around COVID-19 is mostly gone.
So the agency had to come up with new strategies. One of these was to invoke the “bandwagon effect,” which means trying to convince the public that everyone else is getting the shot, so they should jump on the bandwagon.
A poll claiming 72% of those eligible planned to get the new boosters was presented at the CDC’s Advisory Committee on Immunization Practices (ACIP) meeting. But how likely is that to be true?
Only 33% of the population has had a first booster, while 65% have said, “No thanks.” And the interest in COVID-19 jabs is way down — under 5% of preschoolers have received a COVID-19 vaccine in the three months since they were authorized.
The CDC implied to the ACIP that 49% of the public had been boosted, while its own statistics, according to The New York Times, say the actual number is 33%.
Here is how CDC performed the calculation to make it appear the boosters are more popular than they are: 67% of the public is “fully vaccinated,” according to the CDC. Forty-nine percent of those 67% (who are fully vaccinated) equals 33%.
The federal government allocated $1 billion to buy advertising and guarantee positive news coverage (and suppress bad news) to push the earlier COVID-19 vaccines. One wonders how much will be spent to push the new boosters?
According to the CDC, 224 million Americans are “fully vaccinated.” The ACIP members were told that of this number, 210 million are already eligible for the new boosters.
The government has bought 171 million bivalent booster doses so far (105 million from Pfizer and 66 million from Moderna) which can be used for those ages 12 and up.
The FDA and CDC have yet to allow the rollout of new bivalent boosters for children under 12, who in the past received lower-dose COVID-19 vaccines. But the agencies said they plan to do so within weeks.
How long will it work?
Another testy issue for the ACIP committee was the question of how long these boosters will work, and how frequently they will be recommended.
The ACIP members are responsible for giving advice on all vaccines, and they don’t want the COVID-19 vaccines to sour the public on other vaccines.
Although a recommendation to give the bivalent boosters four months after an earlier dose had once been floated, the ACIP committee was asked to approve the boosters when at least two months had passed since a prior dose.
The CDC’s Dr. Evelyn Twentyman said the CDC is no longer counting the total number of doses.
She said that even if a person has received four or five prior COVID-19 vaccinations, a new bivalent booster “should not be denied,” as long as two months have passed since the last dose.
According to the NEJM:
Increased neutralizing antibody titers, as well as clinical effectiveness, have been shown to wane by four months after a third messenger RNA immunization. After a fourth messenger RNA immunization, protection against infection with SARS-CoV-2 Omicron has been reported to wane after just 4 weeks, although protection against severe disease lasts longer.
Hybrid immunity from both vaccination and infection provides greater and more durable protection than either alone.
Four weeks! Antibody titers sink four weeks after the fourth dose — no wonder the CDC is allowing, and may encourage, such frequent boosters.
The COVID-19 vaccinators have coined a new term, hybrid immunity, riffing off hybrid electric cars. It refers to the improved immunity a vaccinated person has if they also got the disease — as if being vaccinated but getting the disease anyway is to be normalized as desirable.
After the CDC spent two years denying that natural immunity — the kind people get after infection — even exists, the agency now is trying to take a lemon vaccine, add natural immunity, call it hybrid immunity and make lemonade!
How was this rollout justified?
Omicron variants have been present since last November, and it was soon discovered that both vaccine-induced and natural immunity due to earlier variants were very limited for Omicron variants, because they are so different from the ancestral strain.
The health agencies and manufacturers have been testing Omicron vaccine prototypes for up to nine months. Most of those tests involved BA.1 and BA.2 Omicron strains.
However, 90% of current cases are caused by Omicron BA.5, which is genetically far from BA.1 and BA.2.
But there was some human data (involving a few hundred subjects each) for several of the earlier Omicron vaccine prototypes, so the health agencies decided to simply pretend that mRNA designed for BA.1 and BA.2 was close enough to BA.5 that the data were comparable.
Since 50% of the vaccine contents would be the old vaccine, the FDA claimed it had already established the safety and efficacy of that half.
Then, to round things out, there were data from mice, which generated comparable antibody levels to the new vaccines as they had to older vaccines. And of course, we can rely on mice to behave exactly like people, right? After all, they have been “humanized” to contain a human ACE-2 receptor.
No, we cannot rely on mice. We cannot even rely on nonhuman primates as a model for vaccines, as every species reacts uniquely and unpredictably to infections and to vaccinations.
But mice data do bulk up the FDA’s authorization “package” so it looks like the agency did a more thorough review.
New boosters not an improvement over old vaccines
Predictions from Nature magazine and Fauci’s Vaccine Research Center at the National Institutes of Health are that the new vaccines will not improve on the old vaccines.
According to the Vaccine Research Center, “A study in nonhuman primates showed that an Omicron specific messenger RNA vaccine was not better than the original messenger RNA-1273 [ancestral Moderna] vaccine for protection against Omicron challenge.”
Nature noted, “An analysis suggests that updated boosters seem to offer much the same protection as an extra dose of the older vaccines — particularly when it comes to keeping people out of hospital.”
Neither of these studies was discussed at the ACIP meeting. No discussion was provided regarding why and how the bivalent vaccines were chosen.
According to the Vaccine Research Center, the Omicron vaccines won’t stimulate a good Omicron response due to antigenic priming, also known as original antigenic sin.
This means the immune system has been programmed to respond over and over again to the first coronavirus infection or vaccine it encountered, even when it encounters different coronavirus antigens later.
How well did the old vaccine work?
The CDC slide below, presented by CDC’s Dr. Ruth Link-Gelles, is not well labeled, but it shows that whether you got two or three doses of the old vaccine, during the Omicron period efficacy in all age groups was under 40% at three months.
By six months it hovered around zero efficacy (no benefit), and after that it was negative (harmful) for most ages.
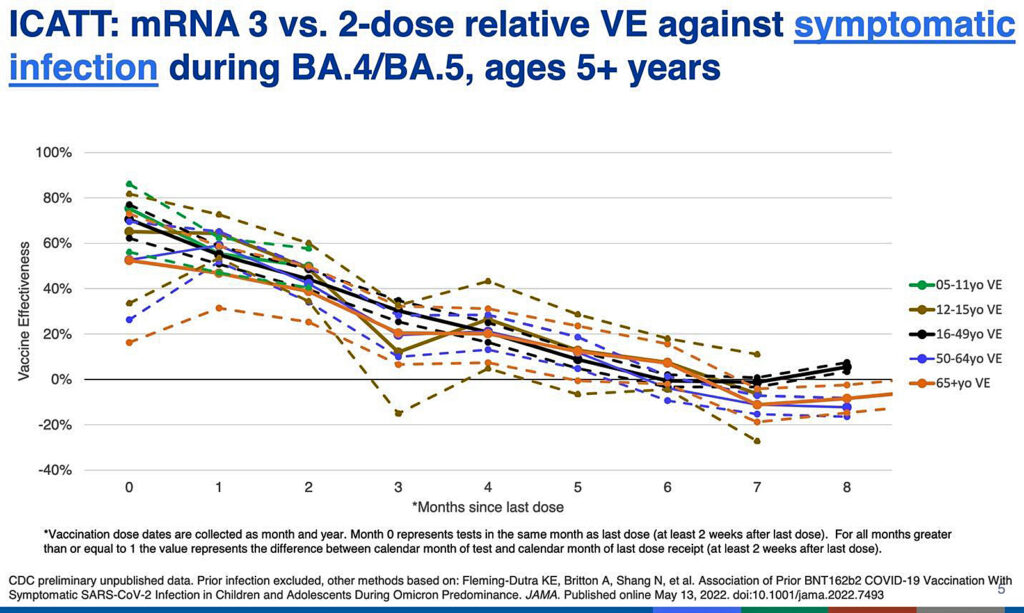
Negative efficacy means the vaccinated are more prone to being infected with COVID-19 than the unvaccinated.
This is consistent with what we are seeing from the U.K. and some other countries: The vaccinated are more likely to get COVID-19.
And it is this effect that public health agencies are probably trying to stave off, or hide, with perpetual boosters.
It appears the public here and in many other countries is being misled to receive an untested (or in other countries a BA.1 or .2 minimally tested) shot on the false promise it will be so much better than the older vaccine.
The regulators know it is unlikely to be better, but their public relations engines are revved up to convince us otherwise.
How safe are the new vaccines?
How safe the new boosters are is anybody’s guess, because you cannot assess human safety from animal models, as they don’t predict the human response.
So what was done to evaluate the safety of the bivalent vaccines?
Reactogenicity: Reactogenicity is a word that refers to short-term vaccine adverse reactions, like fever, redness, fatigue or muscle aches.
According to the CDC briefers, the degree of reactogenicity from the Omicron prototype vaccines was comparable to that from the older, “ancestral” COVID-19 vaccines.
There were no data on more serious side effects, and Dr. Tom Shimabukuro of the CDC said there was no way to assess the risk of myocarditis due to the small number of subjects who received the prototype vaccines.
However, if you look at Pfizer’s chart below, prepared for the ACIP members, you will notice there was greater reactogenicity (more acute side effects) seen after the Omicron prototype vaccines than seen after the older vaccines.
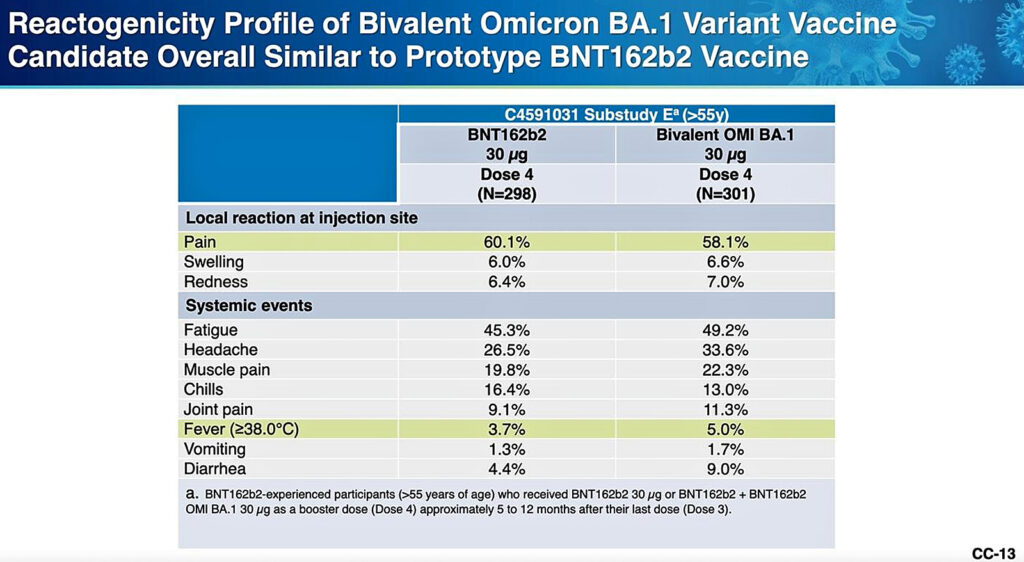
This may be a signal that more severe reactions will result from the newer vaccines, but there is no way to be sure.
Myocarditis: Presenters to the ACIP claimed myocarditis was less common after booster shots of the old vaccine than after the second dose of the initial series.
However, slide 39 (below), shown by Shimabukuro but quickly passed over, showed the opposite.
For 16- to 17-year-old boys and girls, and for men ages 30-39, the chance of myocarditis was increased after a booster.

So there is no reason to think the boosters will be any safer than the second dose, in terms of myocarditis. That risk, by the way, was about 1 in 2,000 young men aged 18-24 after their second dose in one Kaiser study.
Shimabukuro also said if you get vaccinated soon after recovering from COVID-19, increased side effects, at least short-term, are to be expected — but “there is a lack of evidence that it places you at increased risk of myocarditis.”
I am not reassured by the lack of evidence. In fact, pediatric cardiologist Dr. Kirk Milhoan last week reviewed all the evidence that Shimabukuro couldn’t find.
Getting vaccinated soon after recovering from COVID-19 is foolhardy, and any officials mandating the shots after recovery are putting people at even greater risk of adverse reactions, including myocarditis.
Some scientists, including Dan Barouch, M.D., Ph.D., assert that myocarditis is “far more frequent” after a case of COVID-19 than it is after vaccination. But he cited not a single source for this claim.
Dr. Kirk Milhoan, a pediatric cardiologist, reviewed all the recent literature on the question of myocarditis rates after infection versus after vaccination. It appears the vaccine puts you at more risk of myocarditis than a COVID-19 infection does, but there are many different factors that influence risk, including age, gender, whether you already had COVID-19 and how recently and the type of vaccines received.
Moderna vaccines are more likely to cause myocarditis than Pfizer. Receiving a Moderna vaccine after an initial Pfizer vaccine raises the risk even more than getting two Moderna vaccines. (See Table 2 from an important study of myocarditis in four Nordic countries).
France, Germany, Sweden, Norway, Finland, Denmark and Iceland have all halted Moderna COVID-19 vaccinations for young males.
An ACIP member asked whether the Jynneos monkeypox vaccine, which can also induce myocarditis, could be given together with the new bivalent vaccines. Would this increase the myocarditis risk?
The surprising response was, “Read the briefing book,” which may have meant that this was not to be discussed in public.
Should pregnant women get the new boosters?
Speaking of what could be discussed in public, any discussion of pregnancy and COVID-19 vaccination was forbidden at the ACIP meeting. Multiple committee members asked for information on pregnancy, but the briefers steadfastly refused to provide any. Nothing on hospitalizations, deaths, fetal outcomes.
The ACIP members were told they would be briefed on this at a future meeting. Moderna said the company was in the process of enrolling a total of 800 pregnant women in a study — which would someday be completed.
Yet the CDC established a pregnancy registry for the COVID-19 vaccines nearly 18 months ago.
The CDC and FDA must have data on many thousands of pregnancies. Every woman who receives a COVID-19 vaccine dose must provide information on whether she is pregnant before she can be vaccinated and the CDC collects all this information.
Furthermore, there are thousands of Vaccine Adverse Event Reporting System, or VAERS, reports on adverse pregnancy outcomes.
The FDA required Pfizer-BioNTech to study the effect of the vaccine in pregnancy when it issued a license for Comirnaty on Aug. 23, 2021.
The FDA at the same time also required additional vaccine safety studies in children and additional studies on myocarditis. But these studies won’t be completed for up to five years, long after billions of doses have been given and the vaccines will be long out of date.
It is difficult to justify why the FDA would ask for these studies to take so long. Was the agency requesting such long study durations in order to delay its vaccine safety assessment until after the vaccines are no longer in use?
The only conclusion I can draw is that the FDA and CDC don’t like the safety results they already have — and they plan to withhold the bad news for as long as possible.
CDC ‘mum’ on long COVID
The committee was also interested in long COVID. Might the vaccines prevent this dread complication? The CDC was mum.
The CDC briefer claimed the CDC does not have “systematic data” on long COVID. Nor has CDC developed a case definition for long COVID.
Why has CDC delayed investigating this critically important complication?
The New York Times revealed in February 2022 that the CDC conceals the bulk of the public health data it collects. According to the Times, “Much of the withheld information could help state and local health officials better target their efforts to bring the virus under control.”
You are not going to find a more public indictment of our CDC from the Times than that.
Does vaccination fail to prevent long COVD? Does it cause long COVID?
Dr. Paul Marik, an intensive care physician and founder of the Front Line COVID-19 Critical Care Alliance, postulated that both long COVID and many COVID-19 vaccine injuries are due to the same thing: the prolonged presence of spike proteins in the circulation.
If true, there may be considerable overlap between the symptoms and pathology of long COVID and vaccine injuries, and the CDC may be trying to conceal this, or perhaps be seeking a way to claim that all the vaccine injuries are due to COVID-19.
The FDA revoked all Pfizer and Moderna EUAs for the old boosters on Aug. 3.
This was sudden and unexpected. Appointments had to be cancelled, because starting on that date, the old vaccines were limited to use only in young children or for the initial series.
The FDA did not withdraw or recall the licensed Comirnaty and Spikevax vaccines, which also were approved as a booster dose. Is this a tacit acknowledgement that there is no licensed Comirnaty or Spikevax available in the U.S.?
Might the FDA have rolled out the new vaccines so quickly to justify removing most of the old vaccines from use, soon after reports began circulating about their contents containing undisclosed and possibly harmful materials?
This article was originally published by The Defender – Children’s Health Defense’s News & Views Website under Creative Commons license CC BY-NC-ND 4.0. Please consider subscribing to The Defender or donating to Children’s Health Defense.

